Introduction: Chitosan is a linear polysaccharide obtained industrially from deacetylation of chitin particularly interesting for Tissue Engineering (TE) applications. The main of the study was to (i) produce chitosan based hydrogels, (ii) check in vitro vascular and blood cell compatibility and (iii) check in vivo resorption and hemocompatibility.
Methods: Material and cells. Chitosan was purchased from Mahtani, purified and structurally characterized[1]. We produced Ch5% chitosan disks and patches (concentration 5%w/v, degree of acetylation 5% and hydro-alcoholic gelation routes). EPCs were isolated from umbilical cord-blood and cultured[2].
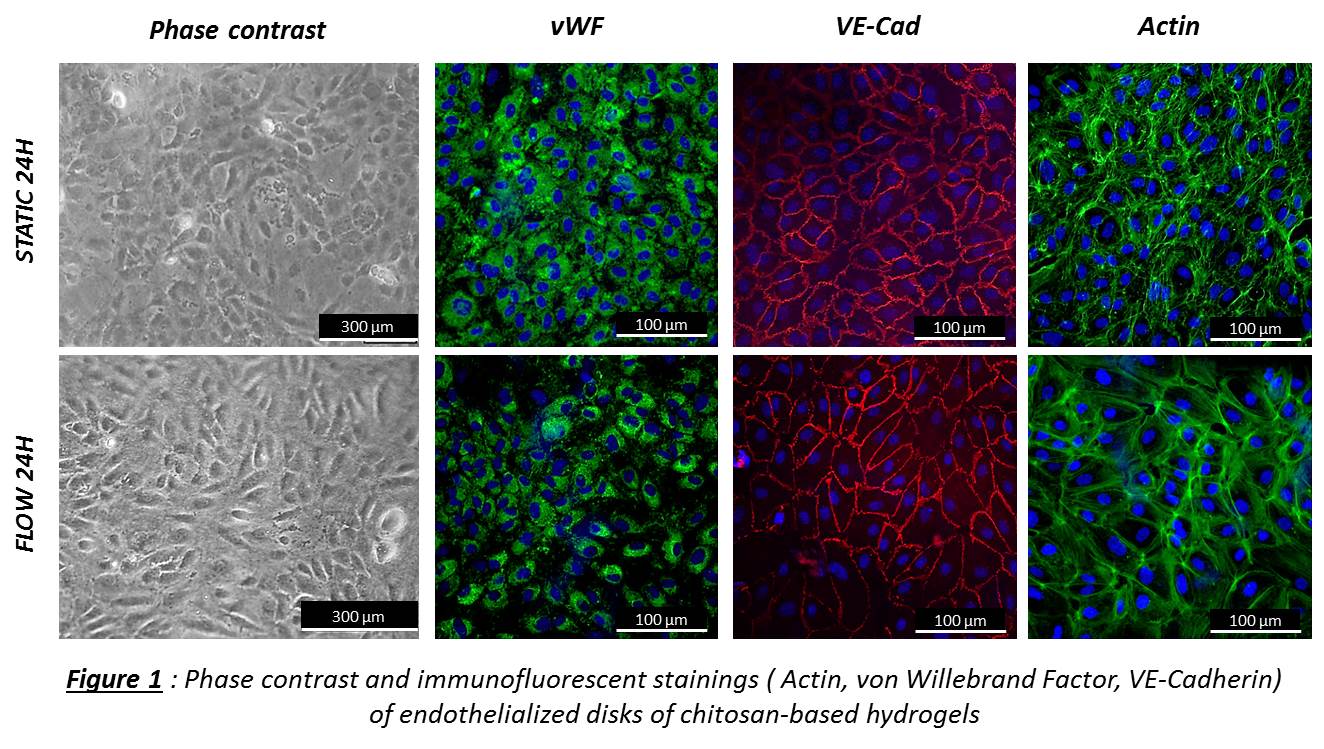
In vitro. EPCs were seeded on Ch5% disks. At 24h, 3d, 7d, 15d, proliferation and viability were evaluated by CyQuant and Live/Dead. EPCs were exposed to shear stress (1.2 Pa) during 8h and 24h in comparison with static conditions. The angle deviation of aligned cells in the flow direction was quantified and stainings for EPC phenotype were performed. Hemocompatibility of Ch5% was evaluated after contact with human blood: partial thromboplastin time (PTT), hemolysis, CH50, C5b-9, C3a and platelet activation.
In vivo. Both subcutaneous and intramuscular implantations of Ch5% were performed in rats (n=3) Longitudinal follow-up was performed by MRI at 10, 30 and 60 days and neovascularization was investigated by μ-CT analysis. Immunohistology (CD 68) were performed after sacrifice. Arteriotomy was performed and repaired with either a Ch5% patch, or a polyester urethane (PU) patch in sheep (n=3) [3]. Patency was assessed peroperatively and every 30 min by carotid Doppler ultrasound (US) exam. Animals were sacrificed at 2h. Tissues including patches were harvested for SEM and histology.
Results and Discussion: In vitro. EPCs were able to grow on Ch5% and confluence was obtained at day 10. When exposed to laminar shear stress (1.2Pa), EPCs aligned in the flow direction at 24H when compared with static conditions and EPC phenotype was maintained (Figure 1). After contact with human blood, a minimal contact activation of the intrinsic pathway of blood coagulation was observed but according to the standard Ch5% was not activator. Then, Ch5% did not induce in vitro hemolysis, nor complement activation, nor platelet activation.
In vivo. In rats, MRI images showed that Ch5% was not degraded at 10, 30 and 60 days after implantation. Ch5% did not seem to induce a chronic inflammation, as shown by immunohistology of CD68 staining. Imaging by μ-CT at days 60 revealed that vessels remain around chitosan without penetrating. In sheep, US showed no flow obstruction inside carotids repaired by Ch5% or control at 2h. SEM results indicated a lower fibrin deposition and platelet aggregation on Ch5% than on PU.
Conclusions: In this study Ch5% elaboration with highly reproducibility and its endothelialization have been shown. Then, in vitro and in vivo results are promising for vascular TE. In the future, in place of patch, chitosan based hydrogel tubes could be cellularized and implanted in large animal model for small diameter vascular TE.
Agence Nationale de la Recherche; LEMI
References:
[1] L. Rami et al. (2014) J.Biomed.Mater.Res.
[2] N.B. Thébaud et al. (2010) J Tissue Eng Regen Med.
[3] M. Rémy et al. (2013) Biomaterials